Identification of novel carbonic anhydrase IX inhibitors using high-throughput screening of pooled compound libraries by DIANA
We have a new paper in the SLAS Discovery. Congrats to Jan, Václav, and the whole team!
Citation: Tykvart, J.; Navrátil, V.; Kugler, M.; Šácha, P.; Schimer, J.; Hlaváčková, A.; Tenora, L.; Zemanová, J.; Dejmek, M.; Král, V.; Potáček, M.; Majer, P.; Jahn, U.; Brynda, J.; Řezáčová, P.; Konvalinka, J. Identification of Novel Carbonic Anhydrase IX Inhibitors Using High-Throughput Screening of Pooled Compound Libraries by DNA-Linked Inhibitor Antibody Assay (DIANA). SLAS DISCOVERY: Advancing the Science of Drug Discovery 2020, 25, 1026-1037. https://doi.org/10.1177/2472555220918836
Abstract: The DNA-linked inhibitor antibody assay (DIANA) has been recently validated for ultrasensitive enzyme detection and for quantitative evaluation of enzyme inhibitor potency. Here we present its adaptation for high-throughput screening of human carbonic anhydrase IX (CAIX), a promising drug and diagnostic target. We tested DIANA’s performance by screening a unique compound collection of 2816 compounds consisting of lead-like small molecules synthesized at the Institute of Organic Chemistry and Biochemistry (IOCB) Prague (“IOCB library”). Additionally, to test the robustness of the assay and its potential for upscaling, we screened a pooled version of the IOCB library. The results from the pooled screening were in agreement with the initial nonpooled screen with no lost hits and no false positives, which shows DIANA’s potential to screen more than 100,000 compounds per day.
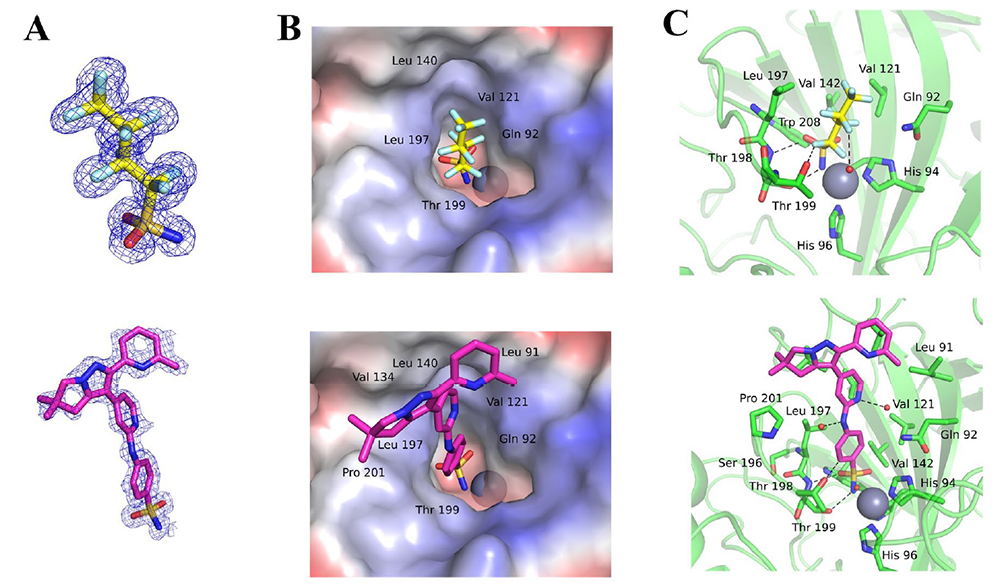
All DIANA screens showed a high signal-to-noise ratio with a Z′ factor of >0.89. The DIANA screen identified 13 compounds with Ki values equal to or better than 10 µM. All retested hits were active also in an orthogonal enzymatic assay showing zero false positives. However, further biophysical validation of identified hits revealed that the inhibition activity of several hits was caused by a single highly potent CAIX inhibitor, being present as a minor impurity. This finding eventually led us to the identification of three novel CAIX inhibitors from the screen. We confirmed the validity of these compounds by elucidating their mode of binding into the CAIX active site by x-ray crystallography.